Α D Glucose and Β D Glucose Are
Glucose D provides all the necessary nutrients and minerals that are required by the body during the days. Glucose C and D are glucose supplements.
/8B2CEC0C484C8C75802585FA0047551F/$file/MA05292_structure.png)
2 5 Anhydro D Mannofuranose 495 75 0 Biosynth Carbosynth
Thus both forms of D-glucose are D--glucose.
/6E13F00C30994BC6802585F90071CCE2/$file/FI09585_structure.png)
. Although many studies on the different aspects of alcoholic fermentation are available in the literature it is still difficult to identify the possible causes of the slowing-down or stuck of fermentations even if the change of some compositional parameters D-glucoseD-fructose and glycerine producedhexoses converted ratios could be assumed as sound signals of a. D-glucose is the enantiomer of the L-glucose and we call it dextrose. Glucose is a widely available monosaccharide and is also known as dextrose and blood sugar.
Your right hand represents D-glucose right-handed sugar if you will and L-glucose left-handed sugar if you will. In a Fischer projection the highest numbered chiral carbon has the OH group pointing to the right. Thus both forms of L-glucose are L---glucose.
Only the composition of vitamins is different from each other. It occurs naturally in free or combined form and it is the most important energy resource for all the organisms. L-Glucose can rotate plane polarized light in anticlockwise direction.
Glucose is the type of pure sugar that has the molecular formula C 6 H 12 O 6. Glucose C is composed of 994 of glucose vitamin C calcium and sodium chloride while glucose D is composed of 994 of glucose vitamin D and calcium. Importance Moreover glucose C helps in refreshing the body and for blood and fluid loss while Glucose D helps in dehydration vitamin D deficiency and cardiovascular and digestive health.
D-glucose and L-glucose are examples of enantiomers. This is C-5 in glucose. Theres no difference between glucose dextrose and d-glucose theyre simply different terms for the same monosaccharide and all have the exact same chemical composition.
Acting as a d-glucose mimic 2-DG inhibits glycolysis due to formation and intracellular accumulation of 2-deoxy-d-glucose-6-phosphate 2-DG6P. L-Glucose is a sugar molecule that is less abundant in nature. Your index finger corresponds to carbon 2 middle finger to carbon 3 ring finger to carbon 4 and pinky to carbon 5.
L-glucose is an equilibrium mixture of α-L-glucopyranose and β-L-glucopyranose. D- and L-sugars are mirror images of one another. Along with fat glucose is one of the bodys preferred sources of fuel in the form of carbohydrates.
They have no scientific difference between them. As 3 Cpda-1 Blood Collection System Dextrose and Electrolyte No. Glucose is one of the main products of photosynthesis and starts respiration.
LOTUS - the natural products occurrence database. Glucose occurs in D-form and L-form. Human GDPGP1 390637 Mouse Gdpgp1 269952 Rat Gdpgp1 308763 cow.
D-Glucose is a sugar molecule that is abundant in nature. It contains six carbons and has an aldehyde group so it is known as an aldohexose. D-Glucose is a natural product found in Gentiana orbicularis Colchicum schimperi and other organisms with data available.
Strictly speaking d- a. Glucose has a chemical formula of C 6 H 12 O 6 and can adopt several structures such as D-glucose or dextrorotary form and L-glucose of laevorotary form. It is a D-glucose and a glucopyranose.
D-glucose is a most commonly occurring isomer of glucose used as a carbohydrate supplementation in case of nutrient deprivation and metabolic disorders such as hypoglycemia. Glucose is mainly manufactured by plants and most of the algae during the process of photosynthesis. In simple terms we can say that it is made up of six carbon atoms twelve hydrogen atoms and six oxygen atoms.
A clockwise rotation is. L-glucose is an organic compound and its IUPAC name is 2S3R4S5S-23456-pentahydroxyhexanal. When glucose is oxidized in the body it.
On the flip side dextrose is the second name for the D-form glucose having the same molecular formula. A Haworth projection representation of the structure of glucose Glucose C6H12O6 is a hexose -- a monosaccharide containing six carbon atoms. People get glucose from bread fruits vegetables and.
The normal concentration of glucose in the blood is about 01 but it may become higher in individuals suffering from diabetes. There is no such thing as L--glucose. Their specific rotations are -1122 and -187 respectively.
For instance D-fructose is l-fructose. A beverage particularly consumed during the summer as a source of instant energy that is said to prevail throughout the day. The ability of 2-deoxy-d-glucose 2-DG to interfere with d-glucose metabolism demonstrates that nutrient and energy deprivation is an efficient tool to suppress cancer cell growth and survival.
On the other hand dextrose can occur only in one optical isomeric form. An important point that none of the other answers have mentioned is that theres a difference between D- and d- likewise L- and l-. Whats most important to know is that because glucosedextrose is only a single molecule it breaks down quickly and is considered a simple sugar.
Coli Metabolome Database ECMDB. The natural form D-glucose is also referred to as dextrose especially in the food industry. As it happens D-glucose and d-glucose are the same isomer but this is not always the case.
D-Glucose is a metabolite found in Escherichia coli strain K12 MG1655. α and D refer to the configurations of different carbon atoms in the molecule. D-Glucose can rotate plane polarized light in the clockwise direction.
Therefore the key difference between glucose C and glucose D is that the glucose contains vitamin C added to it whereas the glucose D contains vitamin D added to it. 75 Dianeal Low Calcium 15 Dianeal Pd-215 Ionosol-MB Isolyte P Leukotrap Normosol-M. In a sugar the D or L designation refers to the configuration of the chiral carbon farthest from the aldehyde or keto group.
Glucose D is an instant energy drink manufactured by Dabur India the largest Ayurvedic medicine and natural products manufacturer. They are non superimposable mirror images of each other. Glucose C 6 H 12 O 6 is a monosaccharide and is a monomer of many polysaccharides such as cellulose starch etc.
In D-glucose three hydroxyl groups and one hydrogen group attach to the right side whereas in L-glucose three hydroxyl groups and one hydrogen group attach to the left side. Glucose can occur in both optical isomers as mirror images.
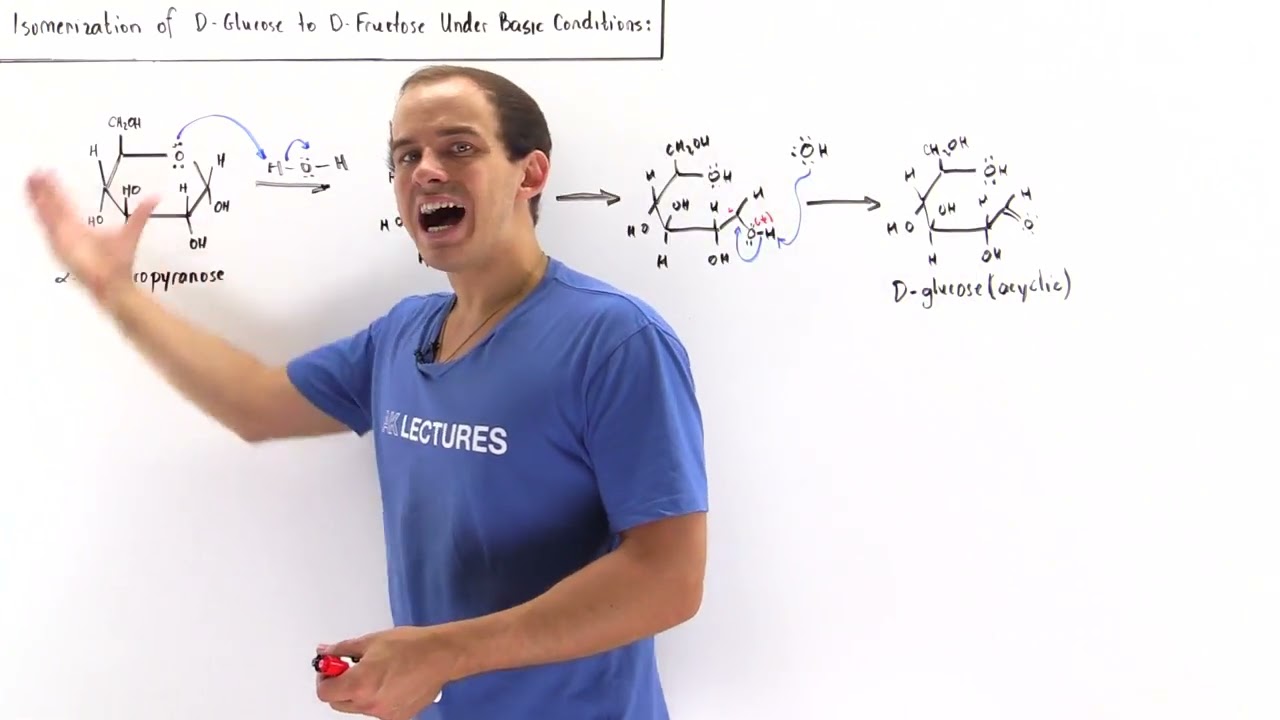
Isomerization Of D Glucose Into D Fructose Youtube

Tell Us How You Feel How Are You Feeling Nursing Education Nurses Week
/6E13F00C30994BC6802585F90071CCE2/$file/FI09585_structure.png)
D Isoascorbic Acid 89 65 6 Biosynth Carbosynth
/852A85AC2CABD2F0802586B7002B61F6/$file/MG08747_structure.png)
D Glucose Anhydrous 50 99 7 Biosynth Carbosynth

Alpha D Glucose Pentaacetate C16h22o11 Pubchem
N Acetyl A D Glucosamine C8h15no6 Chemspider

Study The Structures Of Alpha D Glucopyranose And Beta D Glucopyranose And Mark The Correct Statement
A D Glucopyranose Pentaacetate C16h22o11 Chemspider
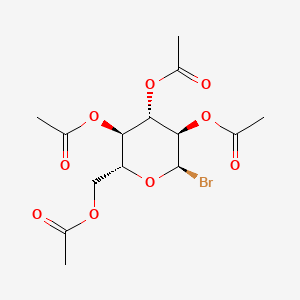
2 3 4 6 Tetra O Acetyl Alpha D Glucopyranosyl Bromide C14h19bro9 Pubchem
/3583740CD8293E90802585F800681DB3/$file/EN03229_structure.png)
4 Nitrophenyl Alpha D Galactopyranoside 7493 95 0 Biosynth Carbosynth
/039A66F36EBE95AC802585FA00474A56/$file/MA04055_structure.png)
D Allose 2595 97 3 Biosynth Carbosynth
/166348B4CC50CDB0802585FA006B6ECF/$file/R-5495_structure.png)
D Ribose 99 0 50 69 1 Biosynth Carbosynth
/0695DC2326282B39802585FA0047F19B/$file/MF00381_structure.png)
D Fucose 3615 37 0 Biosynth Carbosynth
/A5A5CB4DCEAA9277802585FA0046CD40/$file/G-1700_structure.png)
D Galactose 59 23 4 Biosynth Carbosynth
/6BC594FE73705999802585FA00487B43/$file/MP04042_structure.png)
D Pinitol 10284 63 6 Biosynth Carbosynth
/FDAE2D7EB424D885802585FA00486B38/$file/MM46686_structure.png)
4 O Methyl D Glucuronic Acid 4120 73 4 Biosynth Carbosynth
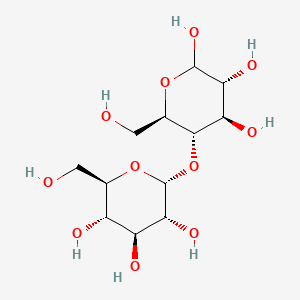
/DFD009C31D799EF8802585FA0047BC28/$file/MD06795_structure.png)
/87767EAA207A0B07802585FA0047AE10/$file/MD04258_structure.png)
Comments
Post a Comment